Published Feb 17, 2021
Discovery's Tardigrades Are Making a Name For Themselves in Our World
Scientists are pulling a Stamets and infusing human cells with tardigrade DNA.

StarTrek.com
In Star Trek: Discovery’s first season, the crew captures a tardigrade-like life form that they name “Ripper.” In “The Butcher's Knife Cares Not for the Lamb's Cry,” Michael Burnham determines that this creature’s unique biology allows it to navigate the mycelial network and Captain Lorca enslaves it for the purpose of piloting the ship.
Exerted to exhaustion, Ripper enters a state of cryptobiosis, forcing Lt. Paul Stamets to take drastic measures: he injects his body with Ripper’s DNA in a desperate attempt to pilot Discovery through the mycelial network himself.
Today, real-life scientists are pulling a Stamets, injecting human cells with tardigrade DNA to confer tardigrade abilities on new life forms.
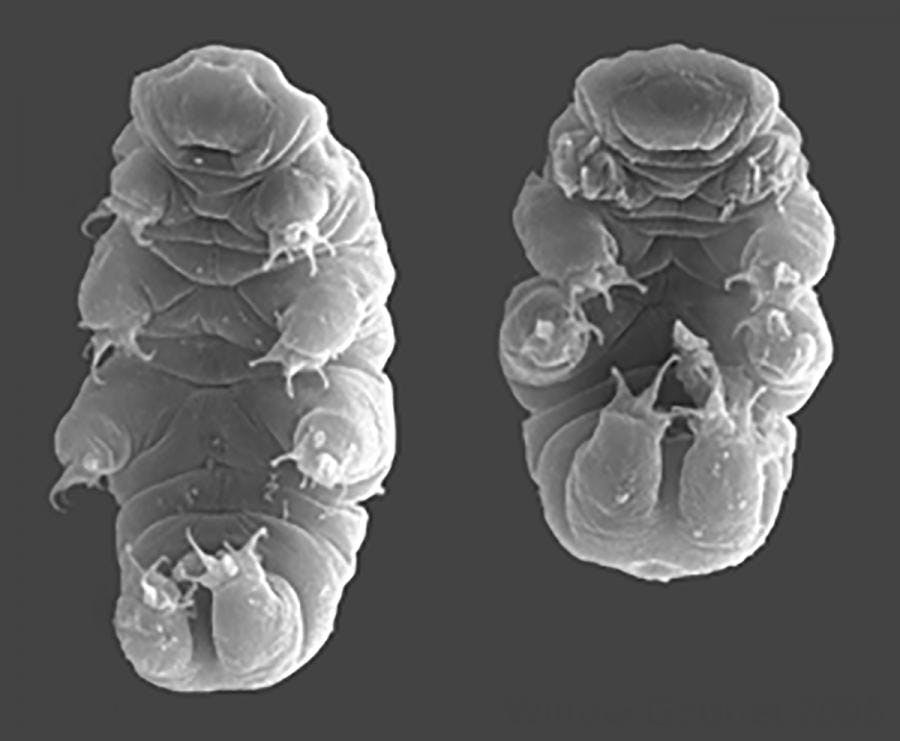
Creative Commons
In the real world, tardigrades are microscopic animals measuring about 1 millimeter in length (or approximately 1/10,000th of Ripper’s size). Don’t let their marshmallow-y looks or their affectionate nicknames (“water bears”, “moss piglets”) fool you — tardigrades are among the hardiest creatures known to science. In fact, scientists have postulated that these tough little critters are the only lifeforms that can handle any planetary apocalypse the universe throws at them. Tardigrades even survived the asteroid that wiped out the dinosaurs.
No need for a global catastrophe just yet — tardigrades can be threatened by a much more mundane event: their humble aquatic homes, such as lakes and tide pools, can dry out. To cope with lean times, tardigrades do, in fact, enter states of cryptobiosis, suspending their metabolic activities and draining their innards of water.
“It’s really an adaptation to a variegated availability of resources, a feast-or-famine lifestyle,” says Christopher Mason, a geneticist at Weill Cornell Medicine (the medical college of Cornell University) and a professed Star Trek fan.
The same dehydration-coping biology also allows tardigrades to survive radiation levels up to a thousand times the lethal dose for humans. This radiation resistance is what present-day scientists are hoping to pilfer from our friendly neighborhood tardigrades.

StarTrek.com
Radiation is dangerous to living tissue because it breaks the chemical bonds in essential molecules, either directly or indirectly by creating harmful radicals, heating, or stirring up the electrical charges on any surface of our biomolecules. DNA is particularly susceptible to radiation damage.
DNA is the instruction manual found in every living cell. A gene is a string of DNA that encodes for the creation of a specific protein. Similar to the crew on a starship, proteins have different tasks that keep the cell functioning smoothly: metabolize nutrients, take out waste, maintain balance, communicate with other cells, replicate, etc. Hence, when DNA is damaged by radiation, the cell’s most basic functions go haywire. For example, cells with mangled DNA can turn cancerous.
But, somehow, tardigrade DNA is impervious to this radiation damage.
The mystery of tardigrades’ superpowers was solved in 2016, when a group of scientists from 10 Japanese research institutions combed through the genes of tardigrades and compared them against all the known genomes of other species. The researchers identified several tardigrade-unique genes, but only one engendered a protein that congregated around DNA. This nondescript protein — named the “damage suppressor” and often shortened to “Dsup” — is the key to the tardigrade’s radioresistance.
The Dsup protein protects DNA from radiation by binding to it and acting as a shield, taking blasts of radiation like a starship’s shields absorb phaser fire. The protein also speeds up the DNA repair process by promoting access to the parts of the DNA chain that are in need of a fix, similar to bookmarking a page with a typo.
Dsup works by enveloping DNA in an electrostatic hug: DNA is intrinsically negatively charged while the Dsup protein is overwhelmingly positively charged. Their bond really is as simple as “opposites attract”—and that’s paramount. All DNA shares the same negative charge, whether it’s inside a tardigrade or a human. Thus, Dsup has the potential to confer the same radio-resistive benefits on a wide range of organisms, if only the cell had the instructions to make it.

StarTrek.com
Inspired by the work of the Japanese researchers, Mason and his lab are transplanting the tardigrade’s Dsup gene into the human genome. They report their findings in a preprint on bioArxiv.
“We're taking, quite frankly, an alien protein and putting it into human cells and seeing what it's doing and how it works,” says Mason.
To transfer the Dsup gene into a human cell’s genome, Mason’s lab first takes a virus and swaps out its original genetic material for tardigrade DNA. This transforms the virus into what is effectively a microscopic hypospray: when the virus breaches the walls of a human cell, it inserts the helpful Dsup gene rather than a deadly viral code. The human cell now has the instructions for churning out protective Dsup proteins as would a tardigrade cell.
Once the Dsup gene has been implanted into the human cells, Mason and his group blasted his cells with X-rays and hydrogen peroxide. Without the Dsup genes, the DNA would shatter. But similar to what the Japanese researchers observed, Dsup-bearing human cells had fewer fragmented DNA bits. These genetically modified cells were also able to grow and function normally, says Mason.

StarTrek.com
What are radio-resistant human cells good for? One intriguing application is space travel. When astronauts venture beyond Earth’s magnetosphere, which protects Earthlings from cosmic rays, a high dosage of radiation becomes a fact of life. Dsup may be key to enabling long-duration space missions by equipping astronauts with cellular protection from this radiation.
“I think we're getting into...a golden era for space research, space biology, aerospace, and medicine,” said Mason, although he acknowledges that we are still far away from an age of tardigrade therapeutics.
While genetic engineering has the potential to bestow enormous health benefits, it also invites a constellation of ethical dilemmas. Star Trek has a storied tradition of dealing with this subject. For example, the 1967 episode “Space Seed” depicts a band of genetically enhanced humans, led by the infamous Khan Noonien Singh, seeking dominance over the rest of humanity on the basis of their “superior abilities.”
Genetic engineering — only a thought experiment for 1960s sci-fi writers — is quickly becoming a reality today. This century, scientists and policymakers must wrestle with questions like: Will access to gene therapy be equitable and just? Where is the line between treatments and cosmetics? Who gets to decide what “defects” need “fixing”?
Mason has several suggestions on how to give reversible radio-protection to human cells that can be administered on demand, side-stepping the ethical conundrum of creating “designer-babies.”
Through triggers that temporarily tack onto DNA, certain genes can be turned on and off like a switch. In this way, Mason speculates that perhaps humans can be equipped with novel genes that temporarily grant radiation resistance and other beneficial traits. While it’s still unclear whether this strategy will work, Mason is hopeful — scientists already have the tools and framework to develop these reversible gene therapies today.
Alternatively, perhaps humans can ingest just a concoction of Dsup proteins as a drug when we need to take up this superpower on a short notice. In this scenario, human genes would be left untampered, and Dsup would be farmed in bacteria that have been genetically engineered to become miniature Dsup protein factories.
If any of these avenues come to fruition, tardigrade DNA will have granted real astronauts greater access to the stars—a striking parallel to how Ripper’s DNA granted Stamets unfettered access to the mycelial network.




